About Us
Our Mission
Recode Biosciences was founded with the singular mission of discovering novel mechanisms to reprogram adult differentiated cells into alternate cells of desired lineage and functional capacity for potential therapeutic use. Our focus is to develop viable, efficient and effective alternatives to embryonic stem cells for therapeutic benefit.
What We Do
We are actively engaged in translating our basic science discoveries into viable therapeutic strategies for a host of human diseases.
Core Platform Technologies

Discovery Platform
Proprietary genomic screening and bioinformatic technology to identify pivotal lineage instructive intrinsic factors in any cell type that are critical for initiation and maintenance of cell lineage commitment.

Transdifferentiation Platform
Proprietary technology to achieve highly efficient transdifferentiation to alternate lineage differentiated cell types without viral or non-viral host genome modification. Because the process utilizes autologous starting cells, derived cells do not suffer immune rejection in in vivo clinical applications.
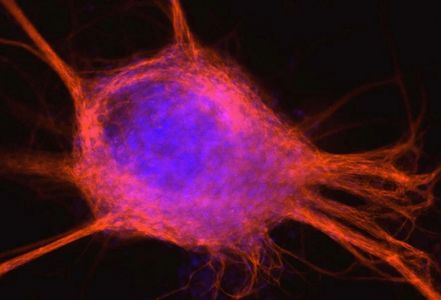
Nuclear Reprogramming Platform
Proprietary technology for high efficiency nuclear reprogramming of starting cells to multipotent or pluripotent stem cells without the need for viral or non-viral host genome modification. Because the process utilizes autologous starting cells, derived cells do not suffer immune rejection in in vivo clinical applications.
clinical applications: wound healing
Tissue Regeneration: Topical Non-viral Biologic Therapeutics

A novel therapeutic strategy is currently in the pre-clinical development stage to derive in situ autologous Mesenchymal Stem Cells in the wound bed through induced dedifferentiation of native in situ wound bed fibroblasts to effect accelerated tissue regeneration, tissue remodeling and wound healing without the need for cell transplantation. This technology promises easier implementation, greater cost effectiveness and lower barrier to entry, even in resource challenged settings e.g. battlezone/frontline hospitals.
Clinical Applications: Degenerative Diseases

Osteoarthritis: Autologous Cartilage Cells for Grafting/Transplantation
A novel therapeutic strategy is currently in the pre-clinical development stage to efficiently and rapidly derive viable autologous cartilage cells ex vivo for in vivo grafting/transplantation in joints affected by osteoarthritis.

Reconstructive Orthopedics: Autologous Bone Cells for Grafting/Transplantation
An exciting new therapeutic strategy is currently in the pre-clinical development stage to efficiently and rapidly derive viable autologous bone cells ex vivo for in vivo grafting/transplantation in Orthopedic and Oral Maxillofacial Reconstructive procedures.

Reconstructive Plastic Surgery: Autologous Fat Cells for Grafting/Transplantation
A highly promising therapeutic strategy is currently in the pre-clinical development stage to efficiently and rapidly derive viable autologous fat cells ex vivo for in vivo grafting/transplantation in Reconstructive Plastic Surgery procedures.
Clinical Applications: Fibrotic Diseases

Pulmonary Fibrosis: Reprogramming Scar Tissue to Lung Parenchyma
A novel therapeutic strategy is currently in the pre-clinical development stage to deliver a targeted biologic drug in vivo in a minimally invasive fashion which triggers reprogramming of pulmonary scar tissue to normal pulmonary cells. Because the proposed therapy does not involve viral/non-viral genetic modification, it does not pose a risk of increased malignant transformation.

Liver Cirrhosis: Reprogramming Scar Tissue to Liver Parenchyma
Am exciting new therapeutic strategy is currently in the pre-clinical development stage to deliver a targeted biologic drug in vivo in a minimally invasive fashion which triggers reprogramming of hepatic scar tissue to normal liver cells. Because the proposed therapy does not involve viral/non-viral genetic modification, it does not pose a risk of increased malignant transformation.
Clinical Applications: Chronic Diseases

Diabetes Mellitus: Autologous Pancreatic Beta Cell Regeneration
A novel therapeutic strategy is in the pre-clinical development phase to deliver a targeted biologic drug in vivo in a minimally invasive fashion to trigger regeneration of native normal pancreatic beta cells in patients with Diabetes. Because the proposed therapy does not involve viral/non-viral genetic modification, it does not pose a risk of increased malignant transformation.

Heart Failure: Autologous Myocardial Cell Regeneration
An exciting new therapeutic strategy is in the pre-clinical development stage to deliver a novel targeted biologic drug in vivo in a minimally invasive fashion to trigger regeneration of native normal myocardium and cardiac remodeling in patients with Heart Failure. Because the proposed therapy does not involve viral/non-viral genetic modification, it does not pose a risk of increased malignant transformation.

Macular Degeneration: Autologous Retinal Regeneration
A highly promising therapeutic strategy is in the pre-clinical development stage to deliver a novel targeted biologic drug in vivo in a minimally invasive manner to trigger regeneration of native normal retinal pigment epithelial cells in patients with Macular Degeneration. Because the proposed therapy does not involve viral/non-viral genetic modification, it does not pose a risk of increased malignant transformation.
Contact Us
Please send us a message or call us for an appointment.
Recode Biosciences, LLC
3926 West Touhy Avenue, Suite 347, Lincolnwood, Illinois 60712, United States